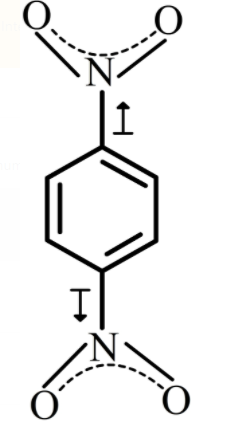
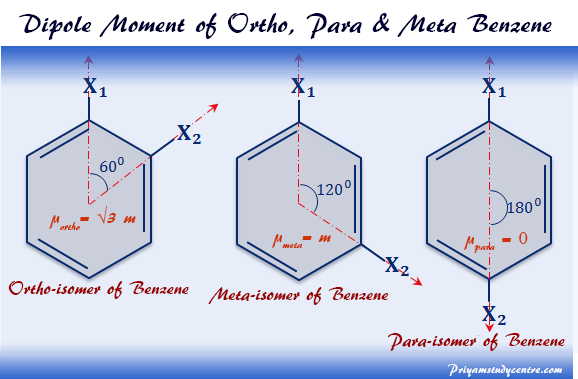
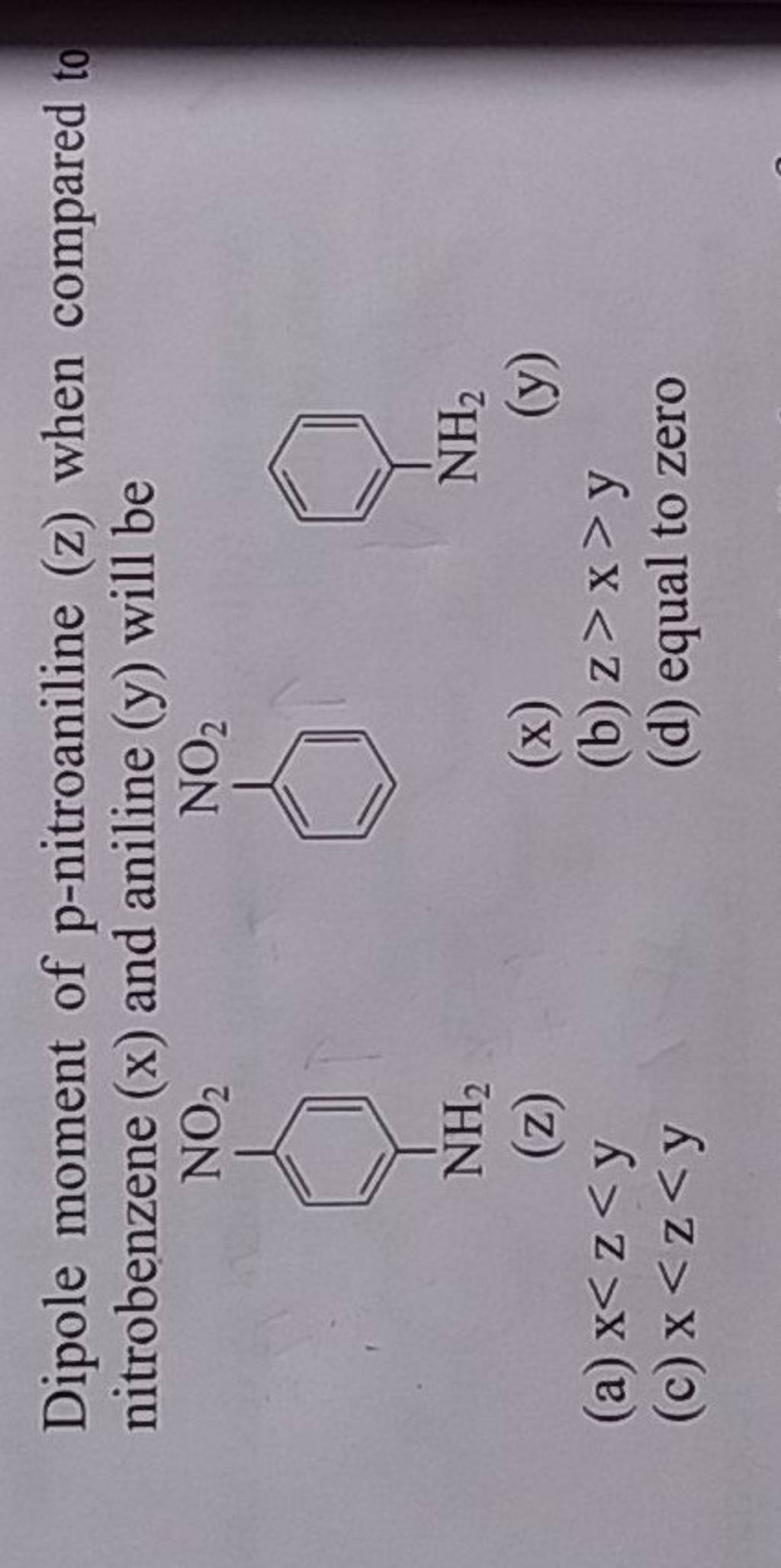
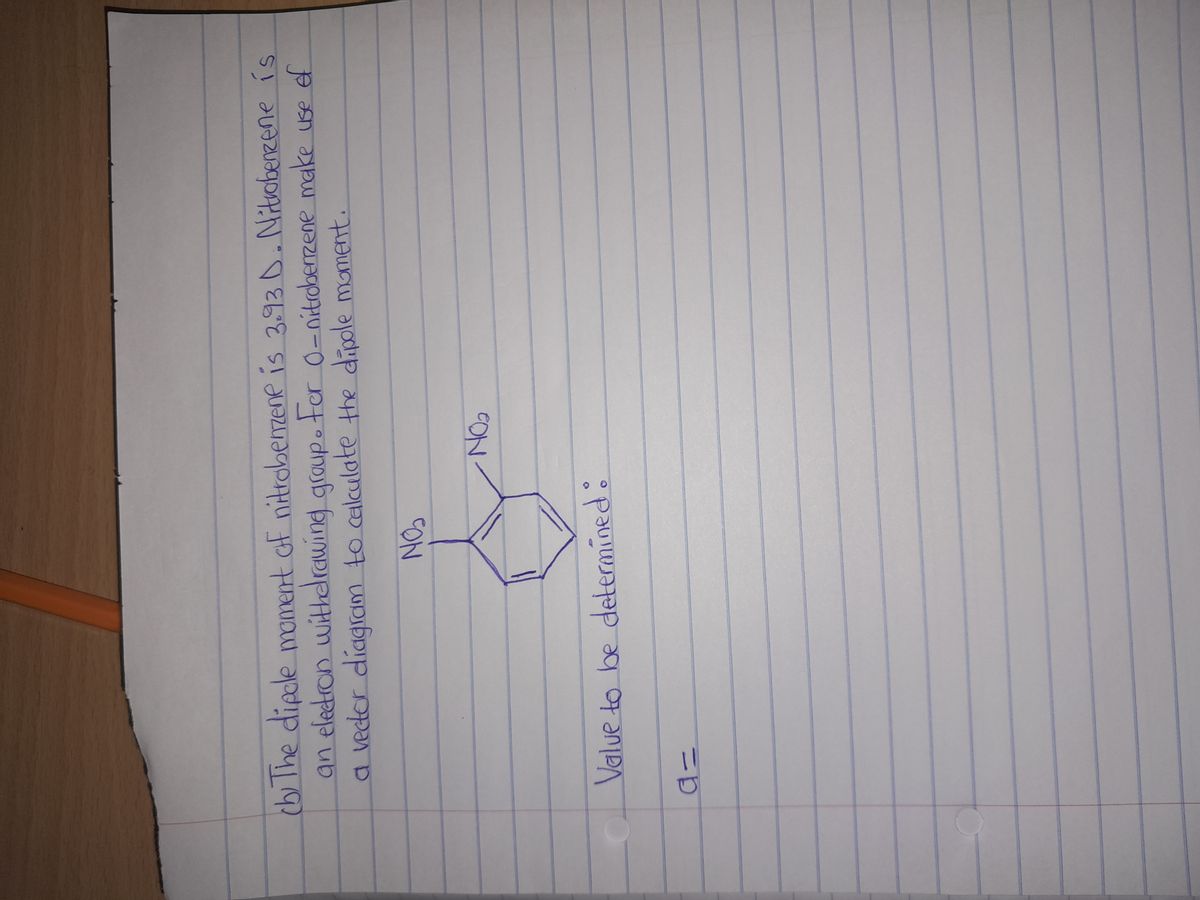
If the dipole moment of Toluene and Nitro - benzene are 0.43 D and 3.93 D respectively, then wha... - YouTube
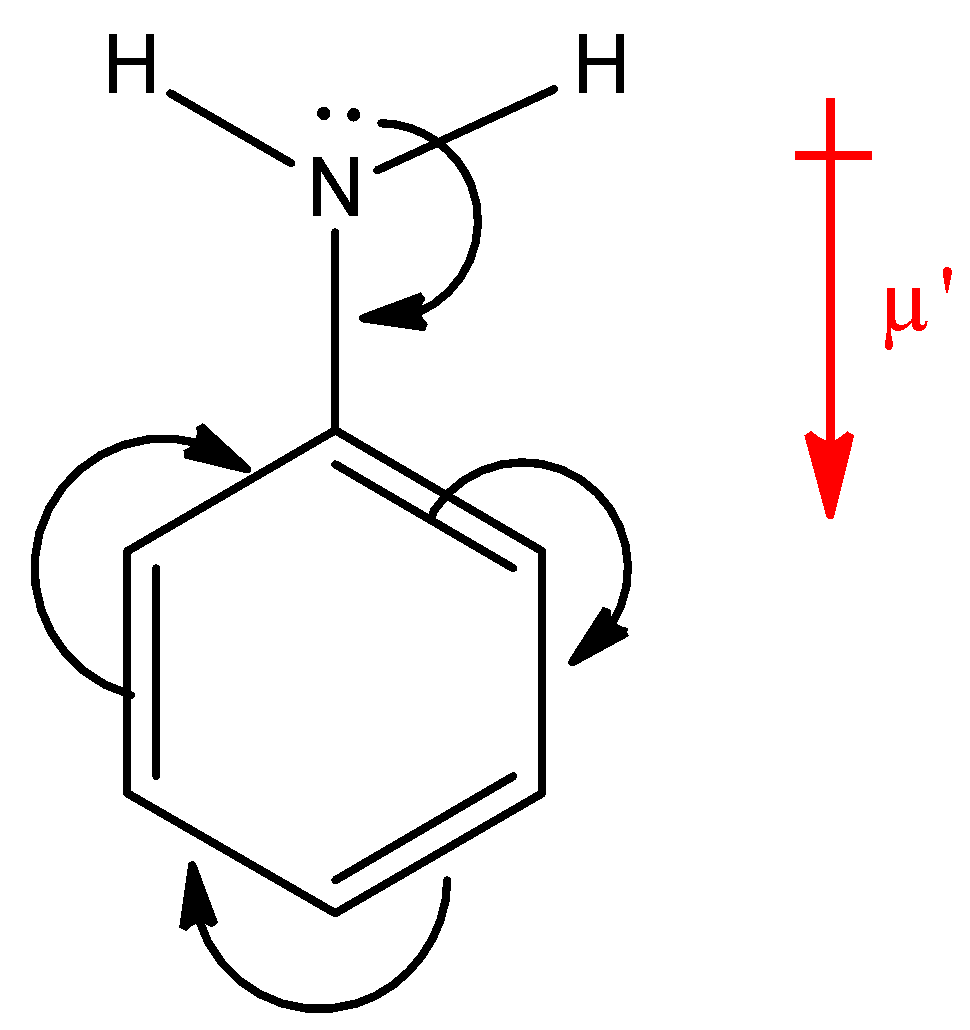
Which compound exhibits maximum dipole moment among the following?(A) \n \n \n \n \n (B) \n \n \n \n \n (C) \n \n \n \n \n (D) \n \n \n \n \n

If the dipole moment of toluene and nitro-benzene are 0.43 D and 3.93 D, then what is the expected dipole moment of p-nitro toluene? : Kaysons Medical

Topic: Dipole Moment Submitted by: Arslan Bashir Department: BS(chemistry) Semester: 8 th Chemistry. - ppt download
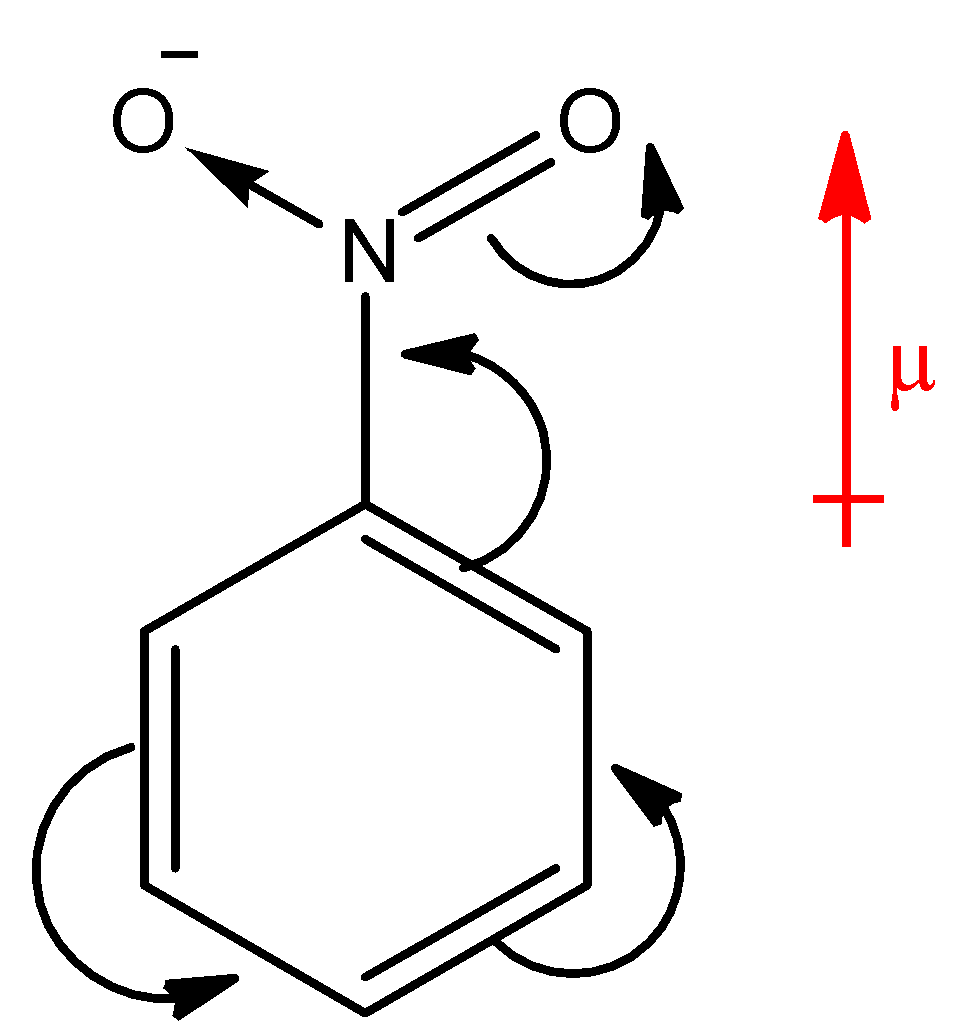
Which compound exhibits maximum dipole moment among the following?(A) \n \n \n \n \n (B) \n \n \n \n \n (C) \n \n \n \n \n (D) \n \n \n \n \n
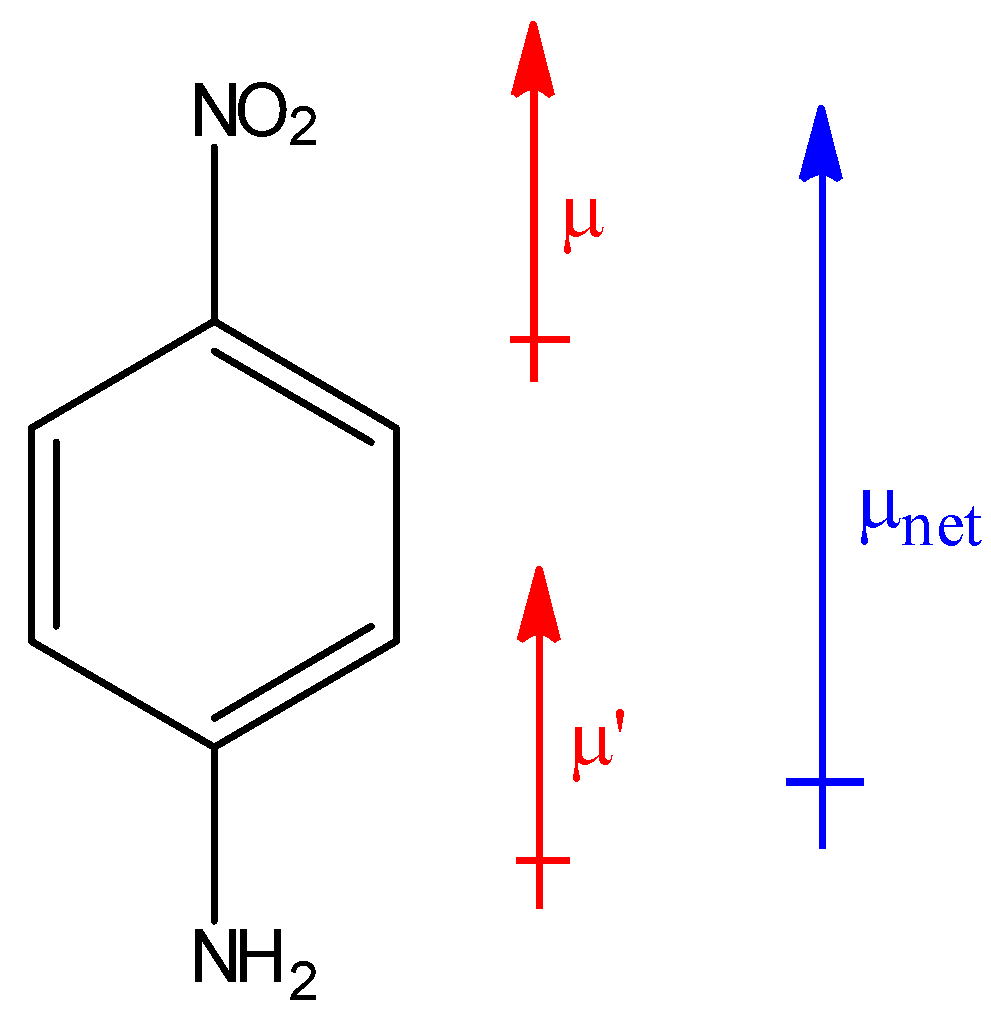
Which compound exhibits maximum dipole moment among the following?(A) \n \n \n \n \n (B) \n \n \n \n \n (C) \n \n \n \n \n (D) \n \n \n \n \n

nitrobenzene has more dipole momment than nitromethane explain - Chemistry - Chemical Bonding and Molecular Structure - 8000237 | Meritnation.com
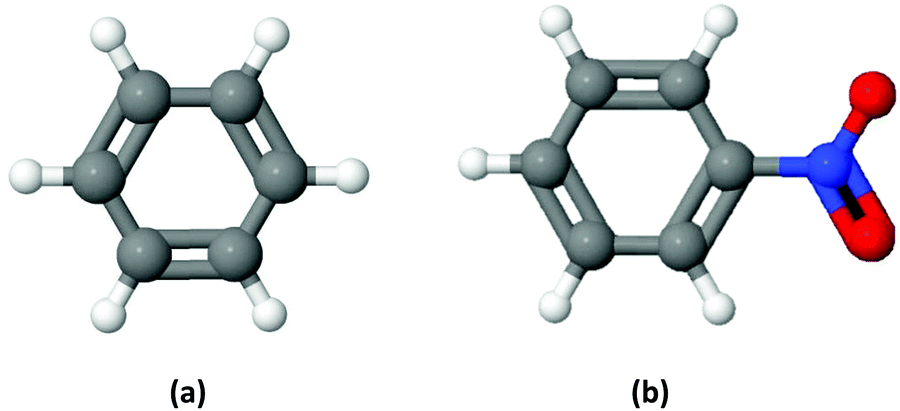
Electron scattering cross sections from nitrobenzene in the energy range 0.4–1000 eV: the role of dipole interactions in measurements and calculations ... - Physical Chemistry Chemical Physics (RSC Publishing) DOI:10.1039/D0CP02039G

49. Which of the following compound will have highest dipole moment? (A) O-nitrophenol (B) p-nitrophenol (C) m-nitrophenol (D) nitrobenzene