![Best Answer] Which has highest dipole moment?? CH3Cl, CH2Cl2, CHCl3 or CCl4. well, I saw a video or Merit - Brainly.in Best Answer] Which has highest dipole moment?? CH3Cl, CH2Cl2, CHCl3 or CCl4. well, I saw a video or Merit - Brainly.in](https://hi-static.z-dn.net/files/df7/fd9f40d6eb64e74ec8cead9b4608b313.jpg)
Best Answer] Which has highest dipole moment?? CH3Cl, CH2Cl2, CHCl3 or CCl4. well, I saw a video or Merit - Brainly.in
✓ Solved: What is a dipole moment ? Give four examples of molecules that possess dipole moments, and...

Draw a three-dimensional representation of the mentioned molecule and indicate the direction of any net dipole for the mentioned molecule. CH3Cl | Homework.Study.com

Why the dipole moment of CH3F is less than CH3Cl although 'F' is more electronegative than 'Cl' ? - CHEMSOLVE.NET | In this moment, Bond length, Chemistry

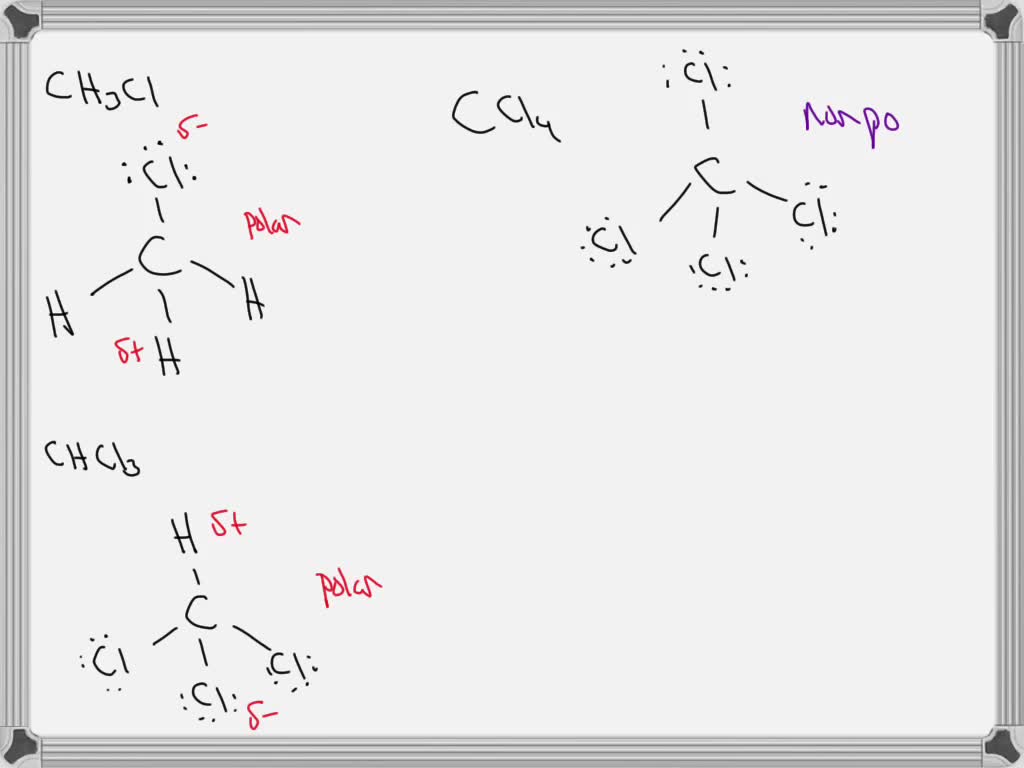
Is the molecule CH3Cl polar or nonpolar? If it is polar, specify the direction of its polarity. | Homework.Study.com

halides - Why does chloromethane have a larger dipole moment than chloroform? - Chemistry Stack Exchange
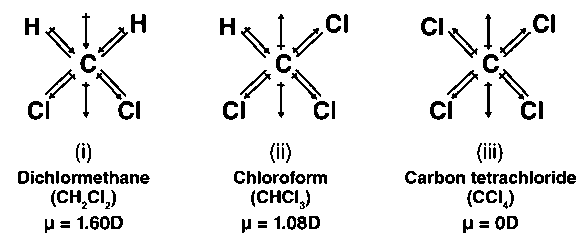
Compare the dipole moment between CHCl_3 and CH_3Cl. Why dipole moment of CH_3Cl is more than that of CHCl_3.

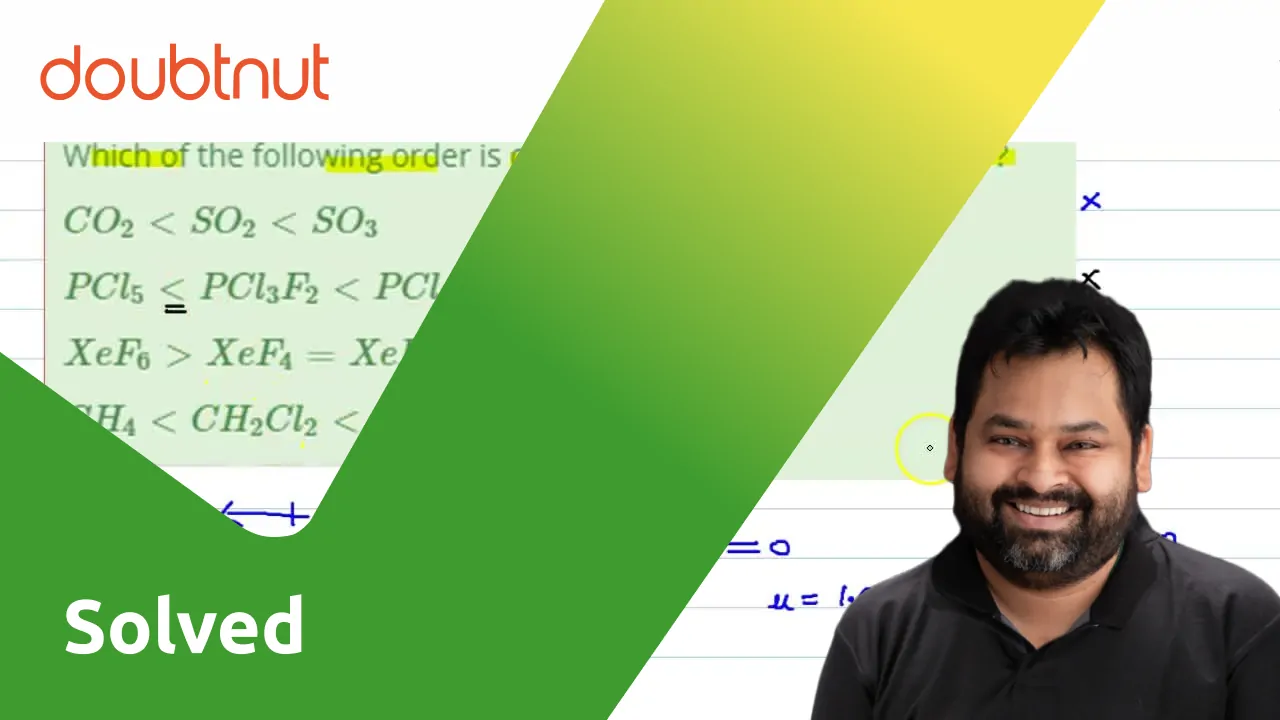
Please explain the dipole moment of CH2Cl2,CH3Cl,CCl4,CHCl3 using diagram( direction )and arrange in increasing order of dipole moment - Chemistry - Haloalkanes and Haloarenes - 16270361 | Meritnation.com
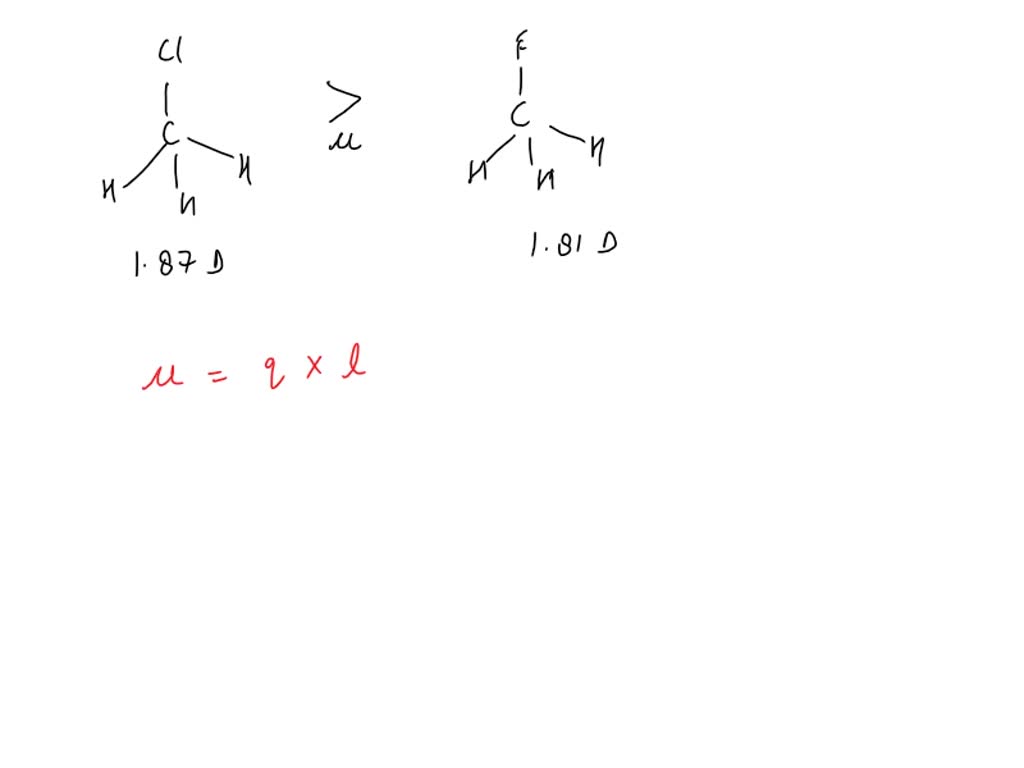
SOLVED: Fluoromethane (CH3F, μ=1.81 D) has a smaller dipole moment than chloromethane (CH3Cl, μ=1.87 D) even though fluorine is more electronegative than chlorine. Explain.
Which of the following molecules has no dipole moment? (a) CH3Cl (b) CHCl3 (c) CH2Cl2 (d) CCl4 - Sarthaks eConnect | Largest Online Education Community